What is relation between Dielectric Constant and Force Between two Charges.
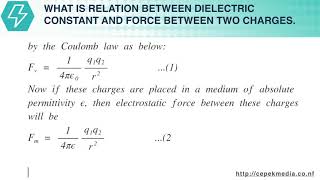
If two charges q1 and q2 are separated by distance r in vacuum then electrostatic force between them will be given by the Coulomb law as below:
Fv=14πϵ0q1q2r2...(1) Now if these charges are placed in a medium of absolute permittivity ϵ , then electrostatic force between these charges will be Fm=14πϵq1q2r2...(2) We know that ϵ=ϵ0ϵr=Kϵ0 where K is dielectric constant or Relative permittivity or specific inductive capacity. or K=ϵϵ0 Now, eqn. (2) becomes, Fm=14πKϵ0q1q2r2...(3) Dividing eqn. (1) by eqn. (3), FvFm=(14πϵ0q1q2r2)(14πKϵ0q1q2r2)=K Hence, dielectric constant (or relative permittivity) of a medium is defined as the ratio of the electrostatic force between two point charges when placed a certain distance apart in vacuum to the electrostatic force between the same charges when placed same distance apart in a medium.
This means a dielectric reduces the force between two charges.
Write your Comment
Please or to post comment!
No comments yet.
MCQ on Optics (Part 1)
MCQ on Optics (Part 2)
MCQ on Optics (Part 3)
MCQ on Optics (Part 4)
MCQ on Optics (Part 5)
Write a note on Charge.