Write a note on Charge.
The number of electrons in an atom is equal to the number of protons, therefore, atom is neutral as a whole. A body consists of atoms, therefore, the body is neutral under ordinary conditions. However, if from such a neutral body, electrons are removed, there occurs a shortage of electrons in the body.
Consequently the body no longer remains neutral. The result is that the body attains positive charge.
Thus, when a body is having shortage of electrons, it is said to be positively charged.
On the other hand, a negatively charged body has excess of electrons from its normal due share.
Total deficiency or excess of electrons in a body is known as charge on the body.
To give a negative charge to any body, extra electrons must be supplied to it.
To supply these extra electrons, work will have to be done, which is stored in the body in the form of energy.
This makes the charged body capable of doing work.
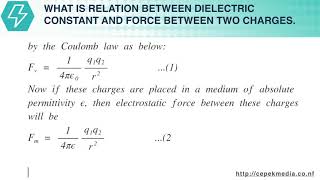
The charge on an electron is so small that it is not possible and convenient to take it as the unit. In practice, the charge is measured in coulomb (C).
1 coulomb of charge = The charge on 625×1016 electrons.
Therefore, practical unit of charge is coulomb.
Like charges repel each other while unlike charges attract each other, means when two like charges are brought near to each other, they will repel each other and if two unlike charges [a +ve and a -ve charge] are brought near to each other, they will start attracting each other.
Write your Comment
Please or to post comment!
No comments yet.
MCQ on Optics (Part 1)
MCQ on Optics (Part 2)
MCQ on Optics (Part 3)
MCQ on Optics (Part 4)
MCQ on Optics (Part 5)
Write a note on Charge.